Climatology : Chapter 3
Earth’s Atmosphere : Role, Composition & Structure
Earths Atmosphere
Basic Facts
- Each planet in the solar system has an atmosphere, a few moons also have it. But only Earth’s atmosphere has a layered structure.
- The Earth’s atmosphere is a complex and dynamic layer of gases that surrounds the planet and are held in place by gravity. It plays a vital role in supporting life and regulating the Earth’s climate.
- High gravity and low temperature support the presence and development of atmosphere
- The layered structure of the atmosphere safeguards our planet from the harmful effects of sunlight, ultraviolet radiation and the solar wind
- 99 per cent of the total mass of the atmosphere is confined to the height of 32 km from the earth’s surface.
- The atmosphere becomes thinner (less denser and lower in air pressure) with increasing altitude. The density decreases, because the gravitational pull decreases with altitude.
- 80 % of the atmosphere is contained within its lowest layer, the troposphere
- No definite boundary exists between the atmosphere and outer space, the area between 100-120 km above the Earth’s surface is considered the boundary.
- Earth’s atmosphere is majorly composed of about 78% nitrogen and 21% oxygen. Other gases, such as argon, carbon dioxide, and trace amounts of various gases, make up the remaining 1%.
- The Earth’s atmosphere plays a crucial role in the greenhouse effect. Certain gases, such as carbon dioxide and water vapor, trap heat from the Sun, creating a stable temperature range necessary for life on Earth.
- The atmosphere acts as a shield, burning up smaller debris and meteoroids before they reach the Earth’s surface, protecting us from potential impacts
- Karman Line
- It is an imaginary boundary between Earth’s atmosphere and the beginning of outer space.
- It is named after the Hungarian-American aerospace engineer Theodore von Kármán, who first proposed the concept
- FAI (Fédération aéronautique internationale) defines it to be at an altitude of 100 kms above the Earth’s surface.
- The Kármán line is defined based on the concept of aerodynamic lift. At altitudes above the Kármán line, the speed required for an aircraft to generate enough lift to sustain flight exceeds orbital velocity. Consequently, vehicles above this boundary need to rely on other methods, such as rocket propulsion, to stay in orbit.
- Such boundary is not universally accepted and is not recognized by any international treaty or organization
Evolution of Earth’s Atmosphere
The details of Earth’s atmospheric evolution are still a subject of ongoing scientific research and investigation. A general overview is mentioned below :
- Early Earth: Around 4.6 billion years ago, after the Earth was formed from a cloud of gas and dust, the atmosphere consisted mostly of hydrogen and helium.
- Outgassing: Due to hot interior of Earth’s, volcanoes and geothermal activity released gases trapped within the planet. These included water vapor, carbon dioxide, nitrogen, methane, etc. This process continued for millions of years and gradually built up the atmosphere.
- Development of Oceans: As the Earth cooled down, water vapor in the atmosphere condensed, leading to the formation of oceans.
- Photosynthesis and Oxygen: Around 2.5 billion years ago, organisms such as cyanobacteria, were capable of producing oxygen through photosynthesis and it resulted in accumulation of oxygen leading to development of an oxygen-rich environment.
- Evolution of Complex Life: The availability of oxygen led to more efficient respiration and energy production, hence the evolution of complex life forms.
- Atmospheric Evolution: Over millions of years, the composition of Earth’s atmosphere continued to change due to Volcanic activity, meteorite impacts, and activities of living organisms. The atmosphere eventually became stable, with a balance between various gases, including nitrogen, oxygen, carbon dioxide, and trace gases.
Concept of Air Pressure
- Air Pressure:
- The air around us carries weight, and it presses everything it touches
- The weight of the atmosphere which exerts pressure on the Earth’s surface, is known as air pressure.
- This force is exerted on Earth’s surface due to the gravitational pull
- Air pressure decreases with increasing altitude due to the decreasing density of the atmosphere
- Air pressure is affected by the mass of air molecules, gravitational pull and the kinetic energy the air molecules carry
- Measurement :
- Air pressure is denoted by the symbol – atm
- One atmosphere (atm) refers to the air pressure at sea level at a temperature of (15°C). This is also the baseline reading
- Meteorologists use a metric unit for pressure called a millibar
- A pressure of 1 atm = 101325 Pa = 1.01325 bar = 1013.25 millibar
- Units of Pressure
- Pa (Pascal)
- The pascal is the unit of pressure in the International System of Units (SI)
- One pascal = pressure exerted by a force of one newton perpendicularly upon an area of one square metre
- 1Pa = 1N/m2 = 1kg/m.s2 = 1J/m3 where N =newton, m =metre, kg =kilogram, s =second, and J =joule
- One atm = 101325 Pa (101.325 kPa)
- bar
- It is also a unit of pressure, and not a part of SI units
- 1 bar = 100,000 Pa ≡ 100,000 N/m2
- One atm = 1.01325 bar = 1013.25 millibar = 101325 Pa
- Pa (Pascal)
Role & Importance of Atmosphere
The Earth’s atmosphere plays a crucial role in supporting life and shaping the planet’s climate.
- Life Support
- The atmosphere contains important gases like nitrogen (78%), oxygen (21%), and traces of other important gases.
- It creates a habitable environment by regulating temperature and distributing heat.
- It creates the pressure without which liquid water couldn’t exist on our planet’s surface.
- Protection from Solar Radiation
- The layered composition of the atmosphere acts as a shield against harmful solar radiation, particularly the ultraviolet (UV) rays
- Ozone absorbs much of the UV radiation, protecting life on Earth
- Climate Regulation
- The atmosphere helps in regulating the Earth’s climate.
- It acts as a blanket, trapping heat from the sun (the greenhouse effect) and preventing it from escaping into space.
- Without the natural greenhouse effect, Earth would be too cold to support life
- Weather Systems
- The atmosphere is crucial to the formation and movement of weather systems.
- Solar energy heats the Earth’s surface, causing air to rise, cool, and condense, leading to the formation of clouds, precipitation, and weather patterns like wind, storms, and hurricanes.
- The atmosphere’s composition and circulation patterns affect the distribution of heat and moisture across the planet.
- Protection from Space Debris
- Earth’s atmosphere acts as a shield against small meteoroids and space debris
- As these objects enter the atmosphere, they burn up due to friction with air molecules
- Communication and Navigation
- The atmosphere enables transmission of radio waves, which is vital for communication systems like radio, television, and wireless technologies
- It also facilitates transmission of signals for GPS (Global Positioning System)
How Atmosphere helps regulate the Earth’s Temperature ?
The Earth’s atmosphere regulates temperature through a combination of processes, including the greenhouse effect, conduction, convection, and radiation.
- Greenhouse Effect
- The greenhouse effect is a natural process in which certain gases in the atmosphere, such as carbon dioxide (CO2), methane (CH4), and water vapor (H2O), trap heat radiated from the Earth’s surface.
- These gases allow sunlight (shortwave radiation) to pass through and warm the Earth’s surface, but they absorb and re-emit the heat energy (longwave radiation) back toward the surface, keeping it warmer than it would be without these gases.
- Conduction
- Conduction is the transfer of heat energy between two objects in direct contact.
- In the lower atmosphere, the surface of the Earth is heated by sunlight, and this heat is conducted to the air molecules in contact with the surface.
- Similarly, during nighttime, the Earth’s surface loses heat to the cooler atmosphere through conduction.
- Convection
- Convection is the transfer of heat energy through the movement of fluids (liquids or gases).
- When the air near the surface is heated, it becomes less dense and rises, creating an updraft. As the warm air rises, it carries heat energy with it.
- At higher altitudes, the air cools and becomes denser, causing it to sink back down to the surface.
- This creates a cycle of rising and sinking air, known as convection currents, which help distribute heat vertically and horizontally in the atmosphere.
- Radiation
- Radiation is the transfer of heat energy through electromagnetic waves.
- The Sun emits shortwave radiation, primarily in the form of visible light and ultraviolet (UV) rays, which penetrate the atmosphere and warm the Earth’s surface.
- In response, the Earth radiates heat energy as longwave radiation back into space.
- However, greenhouse gases in the atmosphere absorb a significant portion of this outgoing longwave radiation, re-emit it in various directions, and a portion of it returns to the surface, contributing to the greenhouse effect and maintaining a stable temperature.
Composition of Atmosphere
The Earth’s atmosphere is primarily composed of a mixture of gases, with traces of other substances such as water vapor, aerosols, and particulate matter. The most abundant are nitrogen (N2) = 78% and oxygen (O2) = 21%. The remaining 1% consists of several other gases and substances
| Constituent Gases | Symbol | Volume Percentage |
| Nitrogen | N | 78.08 |
| Oxygen | O | 20.95 |
| Argon | Ar | 0.93 |
| Carbon Dioxide | CO2 | 0.036 |
| Neon | Ne | 0.002 |
| Helium | He | 0.0005 |
| Krypto | Kr | 0.001 |
| Xenon | Xe | 0.00009 |
| Hydrogen | H | 0.00005 |
- Nitrogen (N2)
- Nitrogen is essential for various biological processes and is a crucial component of proteins and DNA
- Nitrogen gas plays a vital role in the nitrogen cycle, where it is converted into other forms such as ammonia and nitrate, which are necessary for plant growth
- It is a crucial component of amino acids, proteins, and nucleic acids (DNA and RNA), which are the building blocks of life.
- It is an inert gas, meaning it does not readily react with other substances. This inertness helps maintain the overall stability and composition of the atmosphere
- Nitrogen compounds, particularly nitrous oxide (N₂O) and nitric oxide (NO), are greenhouse gases that contribute to the Earth’s radiative balance and climate change. Excessive release of nitrogen compounds from human activities, such as the combustion of fossil fuels and intensive agriculture, can lead to an increase in greenhouse gas concentrations and global warming.
- Nitrogen helps in diluting the activity of oxygen and supports combustion of fuels and food at moderate rate. In absence of nitrogen any fire would have been highly violent and difficult to control
- Oxygen (O2)
- Oxygen is necessary for the respiration of most living organisms, including humans.
- It supports the combustion process and plays a crucial role in the production of energy within cells.
- It is also involved in various atmospheric processes, such as the oxidation of pollutants and the formation of ozone (O3)
- Oxygen participates in various biogeochemical cycles, such as the carbon, nitrogen, and sulfur cycles. In these cycles, oxygen is involved in the transformation and conversion of elements and compounds, enabling the cycling of nutrients within ecosystems. For example, during photosynthesis, plants and algae release oxygen as a byproduct, which then becomes available for other organisms to utilize.
- Argon (Ar)
- Argon makes up approximately 0.93% of the Earth’s atmosphere
- It is primarily sourced from the radioactive decay of potassium-40 in the Earth’s crust
- It is also obtained as a byproduct of the production of nitrogen
- It is used in applications, such as filling incandescent light bulbs.
- Carbon Dioxide (CO2)
- Carbon dioxide is a greenhouse gas and constitutes approximately 0.04% of the Earth’s atmosphere
- Carbon dioxide and water vapour are found only up to 90 km from the surface of the earth.
- Carbon dioxide is released through natural processes like respiration and volcanic activity, as well as human activities such as the burning of fossil fuels and deforestation
- It has a significant impact on the greenhouse effect, contributing to global warming and climate change.
- It is transparent to the incoming solar radiation but opaque to the outgoing terrestrial radiation.
- Carbon dioxide is an integral part of the carbon cycle, a natural process through which carbon is exchanged between the atmosphere, oceans, land, and living organisms.
- Carbon dioxide levels in our atmosphere have risen about 40% since the start of the Industrial Revolution
- Neon (Ne)
- Neon makes up approximately 0.0018% of the Earth’s atmosphere
- Helium (He)
- Helium constitutes approximately 0.0005% of the Earth’s atmosphere
- It is the second lightest element
- It is commonly used for various purposes, including cooling in cryogenics and filling balloons.
- Methane (CH4)
- Methane is a powerful greenhouse gas that is approximately 25 times more effective at trapping heat than carbon dioxide, although its lifespan in the atmosphere is shorter
- Methane emissions can lead to positive feedback loops that amplify climate change. As temperatures rise, permafrost regions in the Arctic and subarctic regions may thaw, releasing methane that has been trapped in frozen soils. Additionally, as the oceans warm, methane hydrates (methane trapped in ice-like structures) can also be released. These feedback loops can further enhance global warming.
- It is produced due to various processes
- Natural Sources
- Decay of organic matter in wetlands
- Wildfires and volcanic activity
- Digestive processes of certain animals
- Anthropogenic sources
- Human activities such as livestock farming, rice cultivation
- Extraction and use of fossil fuels – production and transport of coal, oil, and natural gas
- Landfills
- Burning of biomass
- Natural Sources
- Ozone (O3)
- Ozone primarily occurs in the stratosphere though some ozone, approximately 10% of the total amount, exists in the troposphere
- In the troposphere, ozone is considered a pollutant and contributes to the formation of smog
- In the stratosphere, the ozone layer is mainly found in the lower portion, from approximately 15 to 35 kilometers. The layer of maximum ozone concentration in the stratosphere is referred to as the ozone layer.
- The ozone layer absorbs a significant portion of the sun’s harmful ultraviolet (UV) radiation (UV-B and UV-C rays), protecting life on Earth from its damaging effects
- The absorption of UV radiation by ozone causes a rise in temperature, creating a stable temperature inversion in the stratosphere. This inversion prevents vertical mixing of air, keeping the stratosphere relatively stable and protecting the troposphere below.
- Water Vapor (H2O)
- Carbon dioxide and water vapour are found only up to 90 km from the surface of the earth
- Water vapour decreases with altitude
- It also decreases from the equator towards the poles due to decrease in temperature and low atmospheric moisture
- Water vapor is the most abundant greenhouse gas in the Earth’s atmosphere.
- It absorbs and emits thermal radiation, contributing to the natural greenhouse effect. By trapping a portion of the Earth’s outgoing heat radiation, water vapor helps to maintain a relatively stable and habitable temperature range. It acts as a natural regulator of the climate system.
- When water evaporates from the Earth’s surface, it absorbs heat energy, cooling the surface and the surrounding air. As water vapor rises and condenses to form clouds, it releases this heat energy, warming the atmosphere. This process of latent heat transfer plays a significant role in regulating temperature and climate patterns.
- Water vapor helps fuel the development of storms, such as hurricanes and thunderstorms, by providing the necessary energy for their intensification
- Water vapor is an essential component of the Earth’s hydrological cycle, where it evaporates from bodies of water, condenses to form clouds, and precipitates as rain and other forms of precipitation
- Other Gases
- Earth’s atmosphere also contains trace amounts of Hydrogen (H2), carbon monoxide (CO), nitrous oxide (N2O), sulfur dioxide (SO2), and volatile organic compounds (VOCs)
- These gases are typically present in concentrations of parts per million (ppm) or even parts per billion (ppb), but they can have significant impacts on atmospheric chemistry, air quality, and climate
- Aerosols and Particulate matter
- These include dust, pollen, sea salt particles, volcanic ash, soot, and industrial pollutants
- Convectional air currents may transport them to great heights
- Dust and salt particles act as hygroscopic nuclei around which water vapour condenses to produce clouds.
- They help reflect solar radiation, and can have both cooling and warming effects on the climate
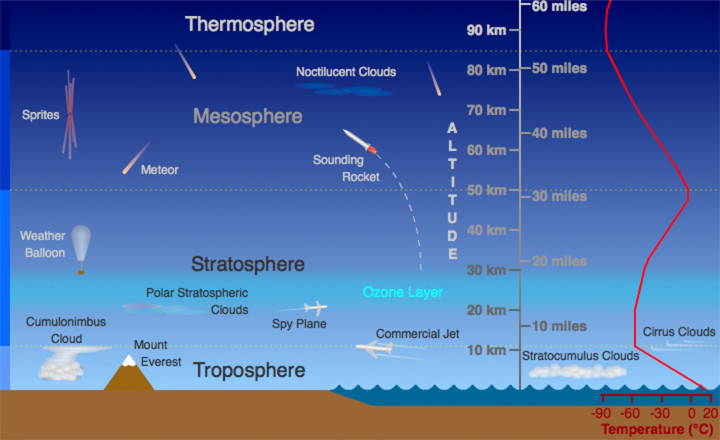
Structure & Layers of Atmosphere
The Earth’s atmosphere is divided into five main layers, each with distinct characteristics and properties.
Troposphere
- The word ‘troposphere’ is derived from the Greek word ‘Tropos’ which means ‘change’. This name indicates turbulence and that all the weather changes occur in this layer
- The troposphere is the lowest layer of Earth’s atmosphere
- It’s average altitude is about 8 to 15 kms. At Earth’s equator it is around 18 km high and at the poles it is about 8 km.
- Thickness of the troposphere is greatest at the equator due to below reasons :
- High insolation and strong convection currents occur over the Equator – Earth is not equally heated everywhere. Heat is transported to great heights by strong convectional currents at the equator, because the equator is warmer. Whereas the poles experience thermal contraction.
- Air is less dense at Equator – The warmer air at the equator is less dense (due to expansion) than the cold air of the poles. Warm air is less dense than cooler air because the gas molecules in warm air have a greater velocity and are farther apart than in cooler air.
- Higher Gravity at Poles – Poles exert more gravitational pull on atmosphere as gravity increases from equator to poles
- Earth’s centrifugal force – It is maximum at Equator, the atmosphere tends to bulge out due to friction and Coriolis force
- Most weather events take place in this layer. It is characterized by vertical mixing of air masses, resulting in the formation of clouds, precipitation, and atmospheric disturbances like thunderstorms, tornadoes, and hurricanes
- In this layer the temperature decreases with increasing altitude at a rate of around 6.5 degrees Celsius per kilometer. This decrease in temperature is known as the lapse rate. Temperature decreases with altitude because
- Primary reason – the troposphere is warmed from below, through absorption and re-emission of incoming solar radiation by the Earth’s surface, rather than being warmed from above by incoming solar radiation.
- Secondly, the decrease in temperature with height is a result of the decreasing pressure. When a parcel of air moves upwards it expands due to lower pressure and we know that air cools as it expands
- The troposphere contains around 75% of total atmospheric mass and is hence the densest layer. It contains majority of Earth’s atmospheric gases, including nitrogen, oxygen, carbon dioxide, water vapor, and trace amounts of other gases
- Air pressure decreases with increasing altitude because at higher elevations, there are fewer air molecules as compared lower levels leading to low density as the air becomes thinner and thinner
- Water vapor content decreases with increasing altitude in the troposphere. This is because
- Temperatures decrease with height and hence rate of water vaporization decreases
- Source of moisture/evaporation is further away
- Water vapours are heavy and at higher altitude, the air density decreases. Thus, it becomes difficult for heavier particles that make water vapours to get vaporized.
- The troposphere contains 99% of the water vapor in the atmosphere.
- The troposphere is where human activities, such as aviation, take place. The reason is that the air in this layer is thinner, making the commercial flight more fuel-efficient.
- The upper boundary of troposphere is known as the tropopause
- Tropopause
- Tropopause marks the end of the troposphere
- It’s height near poles is around 8 km and 18 km above the equator
- In this layer the air becomes dry and devoid of water vapor
- Tropopause is marked by constant temperatures, hence it is called tropopause. It is characterized by a relatively stable temperature inversion, which means that the temperature generally stops decreasing with altitude and starts to remain constant or even increase slightly
- The air temperature at the tropopause is about -80 °C over the equator and about – 45 °C over poles
- Commercial airliners typically fly at altitudes near or just below the tropopause to take advantage of the smoother air and favorable winds found in this region
Stratosphere
- The stratosphere extends from about 10 kms to 50 kms above the Earth’s surface
- The stratosphere contains the ozone layer. Ozone is formed in the stratosphere through the interaction of ultraviolet (UV) radiation with molecular oxygen (O2)
- The temperature in the stratosphere initially remains constant up to 25 km and then gradually increases with altitude. This increase in temperature is primarily due to the absorption of solar radiation by the ozone layer
- Temperatures ranges from −51 °C near the tropopause to −15 °C near the mesosphere
- The stratosphere is relatively stable and dry compared to the troposphere, hence clouds in the stratosphere are rare. However it may experience formation of Polar Stratospheric Clouds (PSCs) and Noctilucent Clouds.
- Polar Stratospheric Clouds (PSCs) : These clouds form in the polar regions during winter, particularly in the high-latitude stratosphere. PSCs are composed of tiny ice crystals and occasionally nitric acid or sulfuric acid droplets. They appear colorful, often displaying iridescent hues, due to the diffraction and scattering of sunlight by the ice crystals.
- Noctilucent Clouds: Also known as polar mesospheric clouds, noctilucent clouds are found in the upper reaches of the mesosphere, but they can occasionally extend into the lower stratosphere. Composed of ice crystals, noctilucent clouds appear luminous and have a distinct silver-blue appearance. They are visible during twilight hours in the summer months at high latitudes.
- The absence of significant weather systems and vertical mixing limits the presence of water vapor, resulting in very low humidity levels
- Commercial jet aircraft typically fly in the lower part of the stratosphere because it provides a smoother ride and consumes less fuel due to reduced air resistance at higher altitudes
- Stratopause
- The stratopause is the boundary between stratosphere and mesosphere, located at an average altitude of 50 kms
- The stratopause is characterized by a temperature inversion, meaning that the temperature stops increasing with altitude and becomes relatively constant or slightly decreases.
- At this boundary, the temperature is generally around -55°C
Mesosphere
- The mesosphere extends from about 50 kms to 85 kms above Earth’s surface
- In this layer the temperature decreases with increasing altitude
- It is the coldest layer of the atmosphere. Near the lower boundary of the mesosphere, temperatures can reach as low as -90°C.
- As you move higher within the mesosphere, temperatures continue to decrease.
- The top of the mesosphere is marked by the mesopause, which is the coldest point in the Earth’s atmosphere. The mesopause has an average temperature around -90°C and is located at an altitude of approximately 85 kms
- The mesosphere is known for the occurrence of noctilucent clouds, also called polar mesospheric clouds. These clouds are thin, faint, and wispy and can be seen during summer months at high latitudes, appearing illuminated by sunlight after sunset or before sunrise. Noctilucent clouds form at extremely high altitudes where the air is coldest.
- The mesosphere is the layer where most meteors burn up upon entering the Earth’s atmosphere. Meteors, also known as shooting stars, are fragments of rocks or debris from space that burn and disintegrate due to the high temperatures generated by atmospheric friction.
- The air density in the mesosphere is extremely low compared to the layers below it. The concentration of molecules is sparse, primarily consisting of oxygen, nitrogen, and traces of other gases such as carbon dioxide and water vapor.
- The mesosphere experiences strong winds and turbulence caused by the interaction of atmospheric waves, gravity waves, and wind shear. This region is responsible for transporting energy and momentum from the lower layers of the atmosphere to higher altitudes.
Thermosphere
- The thermosphere extends from the mesopause to the exosphere, which is the outermost layer of the atmosphere
- It is located at approximately 80 kms above the Earth’s surface and extends upwards to several hundred kilometers
- At such altitudes, the residual gases begin to form layers as per their molecular mass, due to the effect of gravity
- It is characterized by increasing temperatures with altitude.
- The temperature can vary significantly, ranging from hundreds of degrees Celsius to thousands of degrees Celsius.
- However, it is important to note that the thermosphere has a low density, so despite high temperatures, it would not feel hot to a human observer
- The primary source of heat in the thermosphere is intense solar radiation. The thermosphere absorbs high-energy ultraviolet (UV) and X-ray radiation from the Sun, causing the temperature to rise
- The thermosphere is responsible for the formation of the ionosphere.
- The high-energy solar radiation in the thermosphere ionizes neutral atoms and molecules, causing them to lose or gain electrons and become charged particles known as ions.
- The ionosphere plays a crucial role in radio communication and reflects radio waves, enabling long-distance radio communication.
- The thermosphere is the region where the auroras, such as the northern lights (aurora borealis) and southern lights (aurora australis), occur.
- When charged particles from the Sun, known as solar wind, interact with the ionosphere, they can create colorful displays of light in the upper atmosphere.
- The thermosphere has an extremely low density of molecules
- The few particles present in this region are spread far apart, which means that even though the temperature is high, the actual heat energy is low
- Further due to low density this layer free of clouds and water vapor
- The thermosphere is the region where many satellites and spacecraft orbit the Earth. The low density of particles in this layer causes less drag on satellites, allowing them to maintain stable orbits for extended periods.
- The International Space Station (ISS) orbits within the thermosphere at an altitude of around 400 kms. It encounters the effects of the thermosphere, such as increased temperatures during its orbit around the Earth
Ionosphere
- The ionosphere extends from 60 kms to 1000 kms and overlaps the thermosphere and parts of the mesosphere and exosphere
- It consists mainly of a mixture of electrically charged particles known as ions and electrons, and hence called as ionosphere
- Temperature here starts increasing with height
It is primarily composed of ionized atoms and molecules, created through the process of ionization caused by the absorption of high-energy solar radiation, particularly ultraviolet (UV) and X-ray radiation - Radio waves transmitted from the earth are reflected back
- The ionosphere is divided into multiple layers, which are defined by the altitude range and the presence of specific ions. These are D layer, E layer, F1 layer, and F2 layer
- D Layer
- The innermost layer, 48 km to 90 km
- Medium frequency (MF) and lower high frequency (HF) radio waves are significantly attenuated
- This layer disappears at night
- E Layer
- The middle layer, 90 km to 150 km
- It can only reflect radio waves having frequencies lower than about 10 MHz
- At night E layer weakens due to absence of the main source of ionization
- F layer
- Extends from 150 km to 500 km and above
- It has the highest electron density, hence the signals in this layer will escape into space
- It is divided into F1 and F2 sublayers
- F1 Layer
- It is the lower part (150 to 220 km) of F layer and merges into F2 layer at night
- It supports propagation of skywave and long distance high frequency radio communications
- It forms during the daytime due to the absorption of high-energy UV radiation
- The F1 layer is not always present and tends to be more prominent at lower latitudes
- F2 Layer
- Extends 220 to 800 km
- It exists throughout the day and night
- It is the most significant and highly ionized layer within the ionosphere
- F2 layer generally has a higher electron density compared to the F1 layer. The density of free electrons in the F2 layer is usually highest during the daytime when solar radiation is most intense
- Long-range HF radio wave propagation: It can reflect and refract radio waves, allowing them to travel long distances by bouncing off the ionosphere. This phenomenon, known as skywave propagation, is utilized for long-range communication.
- During periods of high solar activity, such as solar flares or geomagnetic storms, the F2 layer can become more ionized, resulting in enhanced ionospheric radio wave propagation conditions
Exosphere
- The exosphere starts at an altitude of about 500 kilometers and gradually transitions into the vacuum of space. However, the exact boundary between the exosphere and space is not precisely defined
- As the altitude increases beyond the exosphere, the density of particles becomes extremely low, and the influence of Earth’s gravity diminishes significantly
- The exosphere is extremely thin and contains very low densities of gas molecules. The density of particles in this region is so low that they rarely collide with each other
- The exosphere primarily consists of atoms and molecules that have escaped the pull of Earth’s gravity. It contains various gases, including hydrogen (H), helium (He), oxygen (O), carbon dioxide (CO2), and traces of other elements
- While the density of particles is low, the exosphere can still have high temperatures. The few particles present in this region can absorb and retain significant amounts of solar radiation, leading to elevated temperatures
- The exosphere plays a role in the generation of auroras and airglow. Charged particles from the magnetosphere interact with the sparse gas particles in the exosphere, causing them to emit light and produce these atmospheric phenomena
Practice MCQs for UPSC Prelims with Answer Key
1. Which layer of the Earth’s atmosphere contains the ozone layer?
a) Troposphere
b) Stratosphere
c) Mesosphere
d) Thermosphere
2.The phenomenon of aurora borealis (Northern Lights) is primarily caused by:
a) Solar radiation
b) Volcanic activity
c) Earth’s magnetic field
d) Air pollution
3.The temperature in the thermosphere:
a) Decreases with increasing altitude
b) Decreases with decreasing altitude
c) Increases with increasing altitude
d) Increases with decreasing altitude
4.Assertion: The ozone layer in the stratosphere is beneficial for life on Earth.
Reason: It absorbs most of the harmful ultraviolet (UV) radiation from the Sun.
a) Both assertion and reason are true, and the reason is a correct explanation of the assertion.
b) Both assertion and reason are true, but the reason is NOT a correct explanation of the assertion.
c) Assertion is true, but the reason is false.
d) Assertion is false, but the reason is true.
5.The layer of the atmosphere where most weather phenomena occur is the:
a) Troposphere
b) Stratosphere
c) Mesosphere
d) Thermosphere
6.Assertion: The mesosphere is the coldest layer of the Earth’s atmosphere.
Reason: The mesosphere is directly above the stratosphere, where the ozone layer is located.
a) Both assertion and reason are true, and the reason is a correct explanation of the assertion.
b) Both assertion and reason are true, but the reason is NOT a correct explanation of the assertion.
c) Assertion is true, but the reason is false.
d) Assertion is false, but the reason is true.
7.The layer of the atmosphere that contains the ionosphere is the:
a) Troposphere
b) Stratosphere
c) Mesosphere
d) Thermosphere
8.Assertion: The troposphere contains approximately 75% of the total mass of the Earth’s atmosphere.
Reason: The troposphere is the closest layer to the Earth’s surface and contains most of the air we breathe.
a) Both assertion and reason are true, and the reason is a correct explanation of the assertion.
b) Both assertion and reason are true, but the reason is NOT a correct explanation of the assertion.
c) Assertion is true, but the reason is false.
d) Assertion is false, but the reason is true.
9.The layer of the atmosphere where meteors burn up upon entering is the:
a) Troposphere
b) Stratosphere
c) Mesosphere
d) Thermosphere
10.Assertion: The Earth’s atmosphere is primarily composed of nitrogen.
Reason: Nitrogen makes up approximately 78% of the Earth’s atmosphere by volume.
a) Both assertion and reason are true, and the reason is a correct explanation of the assertion.
b) Both assertion and reason are true, but the reason is NOT a correct explanation of the assertion.
c) Assertion is true, but the reason is false.
d) Assertion is false, but the reason is true.
11.The layer of the atmosphere where the International Space Station (ISS) orbits is the:
a) Troposphere
b) Stratosphere
c) Mesosphere
d) Thermosphere
12.Assertion: The thermosphere is the layer of the atmosphere with the highest temperatures.
Reason: The thermosphere directly absorbs the majority of the Sun’s ultraviolet radiation.
a) Both assertion and reason are true, and the reason is a correct explanation of the assertion.
b) Both assertion and reason are true, but the reason is NOT a correct explanation of the assertion.
c) Assertion is true, but the reason is false.
d) Assertion is false, but the reason is true.
13.The layer of the atmosphere that protects the Earth from most of the Sun’s harmful ultraviolet radiation is the:
a) Troposphere
b) Stratosphere
c) Mesosphere
d) Thermosphere
14.The layer of the atmosphere that is characterized by decreasing temperatures with increasing altitude is the:
a) Troposphere
b) Stratosphere
c) Mesosphere
d) Thermosphere
Answer Key
- b) Stratosphere
- c) Earth’s magnetic field
- c) Increases with increasing altitude
- a) Both assertion and reason are true, and the reason is a correct explanation of the assertion.
- a) Troposphere
- b) Both assertion and reason are true, but the reason is NOT a correct explanation of the assertion.
- d) Thermosphere
- a) Both assertion and reason are true, and the reason is a correct explanation of the assertion.
- c) Mesosphere
- a) Both assertion and reason are true, and the reason is a correct explanation of the assertion.
- d) Thermosphere
- b) Both assertion and reason are true, but the reason is NOT a correct explanation of the assertion.
- b) Stratosphere
- a) Troposphere